Abstract
Background: Peripheral T-cell lymphomas (PTCLs) are uniquely sensitive to epigenetic modifiers. Our group previously demonstrated that combinations of epigenetic modifiers such as histone deacetylase inhibitors (HDACi), hypomethylating agents and pralatrexate produced potent synergy in pre-clinical models of PTCL, which produced compelling activity in early phase clinical studies. We have demonstrated that epigenetic modifiers, such as decitabine and 5-azacytidine (Marchi et al; Br. J. Haematol. 171, 2015) induces the expression of cancer testis antigens and established that pralatrexate acts as an immunomodulatory agent affecting genes involved in cytokine production, viral response and apoptosis. These pre-clinical data suggest a role for the incorporation of the immune-checkpoint inhibitor, pembrolizumab, to epigenetic backbones. Herein, we report on the differential clinical activity of adding the immune checkpoint inhibitor, pembrolizumab, to decitabine, pralatrexate, or the combination and we describe the role of cytokines as a biomarker of treatment response.
Trial Design & Methods: This is a phase 1b study of pembrolizumab combined with pralatrexate alone (Arm A), with pralatrexate + decitabine (Arm B), or decitabine alone (Arm C) in patients with relapsed and refractory PTCL and CTCL. A standard 3+3 dose-escalation was applied in the doublet Arms (A and C) while a DLT-adapted partial order continual reassessment method (POCRM) for dose-finding with combinations of agents was applied in the triplet Arm (Arm B). We evaluated the cytokine profile using a Luminex-based immunoassay on serum samples obtained from trial patients on days 1, 8 and 15 during cycle 1 of treatment. Quantitative changes in 25 unique cytokines were evaluated, and these changes were correlated with clinical outcomes.
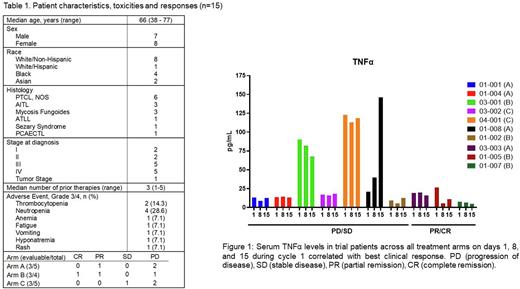
Results: We enrolled 15 patients with six patients in Arm A, four in Arm B, and five in Arm C. All patients that received at least one dose of drug were evaluable for toxicity. One dose limiting toxicity (DLT) was observed in Arms A and B (grade 3 thrombocytopenia and febrile neutropenia, respectively). In Arm C, three DLTs were observed including one patient with grade 3 hyponatremia and rash; one patient with grade 4 thrombocytopenia, neutropenia, and anemia; and one patient with grade 4 neutropenia. There were no treatment related deaths. Nine patients out of 15 were evaluable for response at the time of this analysis. Of these nine evaluable patients, one achieved a complete remission (Arm B), two achieved partial remission (1 in Arm A, 1 in Arm B), one had stable disease (Arm C), and five experienced disease progression (2 in Arm A, 1 in Arm B, and 2 in Arm C). Interestingly, two of the three responses were seen in patients who received the triplet of pralatrexate, decitabine, and pembrolizumab (Arm B) and one response was seen in a patient treated with pralatrexate and pembrolizumab (Arm A). The serum cytokine profile revealed significant changes in only five of the 25 cytokines surveyed. Of the five cytokines, three pro-inflammatory cytokines, TNFα, MIP-3α and IL-10, measured at day 1 pre-treatment exhibited significantly higher levels (p= 0.0001, 0.0008 and 0.003 respectively) compared to healthy controls. Trial patients who responded to treatment (PR/CR) in either Arms demonstrated a decline in serum levels of TNFα and IL-10 by day 15 of cycle 1, whereas non-responders (PD/SD) showed stable to increased levels of both TNFα (Figure 1) and IL-10.
Conclusion: These preliminary data suggest that the integration of pembrolizumab into an epigenetic backbone is safe and demonstrates encouraging responses in heavily pretreated patients with PTCL and CTCL. Pharmacodynamic data suggests that certain cytokines might have predictive value as biomarkers of disease response and progression. Interestingly, patients that responded (PR/CR) to the study drugs displayed a decline in cytokine levels, highlighting the prognostic implications of these cytokines. Additional pharmacodynamic studies, including flow cytometry of peripheral blood mononuclear cells, and pharmacokinetic studies are ongoing. Clinical trial registry NCT03240211.
Disclosures
Jain:Abcuro, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Acrotech LLC: Research Funding; Crispr Therapeutics: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Mersana Therapeutics: Research Funding; Myeloid Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; SecuraBio: Membership on an entity's Board of Directors or advisory committees; SIRPant Immunotherapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Williams:Janssen: Consultancy, Research Funding; Kite Pharma: Consultancy; Kymera: Consultancy, Research Funding; Pharmacyclics: Research Funding; International Oncology Network: Honoraria; Gilead: Consultancy; Astra Zeneca: Consultancy; Research to Practice: Honoraria; TG Therapeutics: Consultancy. O'Connor:TG Therapeutics: Current Employment, Current equity holder in publicly-traded company. Manavalan:Astex: Research Funding. Marchi:University of virginia: Patents & Royalties: 3062/170 PROV; Myeloid Therapeutics: Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Celgene/BMS: Research Funding; Astex Pharmaceuticals: Research Funding; NomoCan Pharmaceuticals: Research Funding; Kyowa Kirin: Honoraria; Daiichi Sankyo: Other: Participation at advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal